Introduction
Recently evidence was provided that a thyroid gland as a link of the hypothalamo-pituitary-thyroid axis contributes to the neuroendocrine adaptational changes initiated by the hypothalamo-pituitary-adrenocortical axis under stress conditions. It was demonstrated that stress provoked fluctuation of the thyroid hormones level and structural changes in the follicular compartment of the thyroid gland [1,5]. It was also shown that stress might produce prominent and irreversible changes in the thyroid gland including development of the autoimmune thyroid pathology [6] but age-related aspects of the problem remained underestimated. In rats development of a thyroid gland continues during the first three weeks of postnatal development, and after that it enters its maturation phase during weaning period [3]. Development of the thyroid gland in rats of preweaning age is influenced by a surge of sex steroids in blood which effects its maturation either directly or by implication through the thyroid stimulating hormone [2], which provides complicated interaction between hypothalamo-pituitary-adrenal and thyroid axes in stress reactions of the growing body.
Objective
The objective of the present investigation was to evaluate age-related modulation of the microscopic changes in the follicular compartment of the thyroid gland under mild chronic stress conditions.
Total of 48 Sprague Dawley rats aged 14, 21 and 30 days after birth were involved in this research, with 16 animals per age group, out of which 8 animals were exposed to the mild chronic (restraint) stress [8] in a plastic box for 5 hours a day continuously for 7 days, while the other 8 rats served as an age-matched control. Animals were kept in the standard animal house conditions with access to water and food ad libitum.
Thyroid gland of the control and experimental animals was sampled, fixed in formalin, embedded in paraffin. According to the recommendations [5] serial histological sections were stained with hematoxylin-eosin, and those with the largest section area were selected for image analysis. Five random microscopic fields were taken for evaluation under 10x objective. Captured images of the thyroid gland were saved under .tiff extension, recorded for blind evaluation by the two investigators and quantified by image analysis. The contours of the follicles were outlined, images were calibrated and measurements of the area, perimeter and diameters (maximal and minimal) of the follicles and the height of the follicular epithelial cells were performed using Leica QWin software (Leica imaging systems, Cambridge, UK). The measurements were transported to the Excel software with subsequent estimation of the circularity index of the follicles, area of the follicular epithelium and thyroid activation index [1]. The significance of the differences in these parameters in control and experimental animals were assessed by the Student´s test. The level of significance was set at p < 0.05.
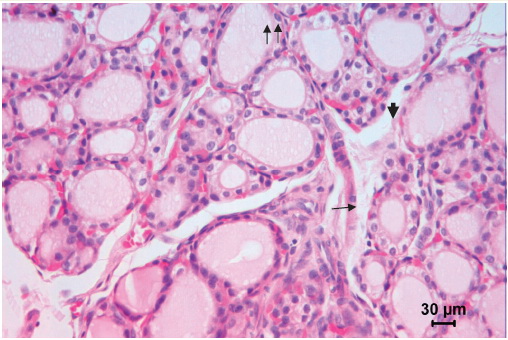
Figure 1. Thyroid gland of a 30-day old control rat. Middle-sized follicles dominate. Epithelial lining is cuboidal or low columnar with nuclei in the basal part of the cells (arrow). Apical pole of some thyrocytes is protruding in the follicular lumen (arrowhead). Parafollicular cells are visible in the epithelial lining of the follicles (double arrow). Microphotograph, hematoxylin-eosin stainig.
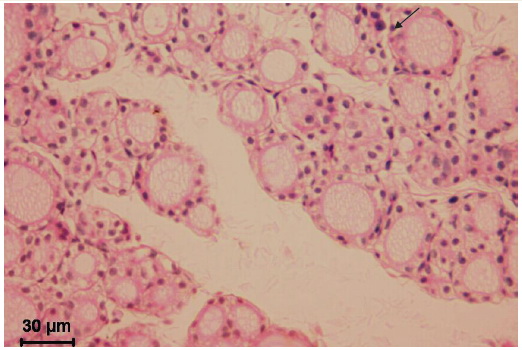
Figure 2. Thyroid gland of the 30-day old rat after mild chronic stress. Small follicles are dominating with low columnar and cuboidal lining. Colloid is light oxyphilic and foamy, resorption vacuoles are numerous (arrow). Interfollicular tissue volume is increased. Microphotograph, hematoxylin-eosin staining.
Results
In preweaning and weaning control animals follicular compartment of the thyroid gland is filled with small and middle-sized follicles lined mostly with cuboidal epithelium. In some of the follicles epithelial lining was low columnar or squamous. Smaller follicles with higher epithelium were concentrated in the center of the section while larger follicles with lower thyrocytes were located mainly at the periphery. Large follicles were not typical for this age group. Follicles are filled with homogenous light oxyphilic colloid. Resorption vacuoles were common in both small and middle-sized follicles. Apical protrusions of the epithelium were not seen. In infant rats the follicles looked larger while the large follicles were observed mainly at the periphery of the gland. Resorption vacuoles were present in the follicles of different size. In this age group the follicles were more heterogenous in size and contained more colloid.
Exposure to chronic stress resulted in lower body weight, thymic involution and adrenal hyperplasia in the experimental animals of all the age groups. In the thyroid gland stromal proliferation and microcirculatory lesions could be seen. The follicles of the thyroid gland demonstrated heterogeneity in size and shape. The follicular epithelium remained mainly cuboidal or low columnar with persisting resorption vacuoles in all the age groups.
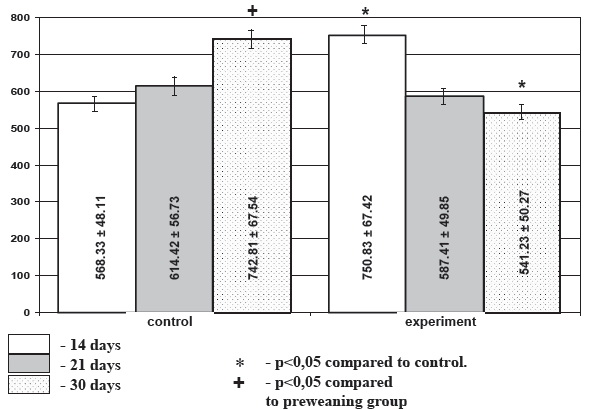
Diagram 1. Area of colloid in the thyroid follicles in chronic stress (mcm2), М+/-m.
Morphometric investigation demonstrated that under chronic stress conditions the area of follicles of the weaning and infant rats showed 14,2% and 11,4% decrease in experimental rats accordingly compared to the age-matched control. In preweaning rats the follicular area increase under stress exposure comprised 6,7%, though this difference did not reach level of significance. Under stress conditions height of the follicular epithelium was decreased insignificantly in preweaning and weaning animals (21,2% and 7,2% accordingly) and was increased (9,4%) in the infant animals. Area of colloid was significantly higher in the preweaning stressed rats and significantly lower in the infant animals (p<0.05) (diagram 1) while in the weaning animals the decrease in colloid area was not meaningful. Activation index of the thyroid gland was decreased in the weaning (p>0.05), and preweaning (p<0.05) stressed rats. It was significantly increased in the infant experimental animals (p<0.05). Thus among the selected morphometric parameters of the thyroid gland mean area of the colloid and the thyroid activation index proved to be the most informative.
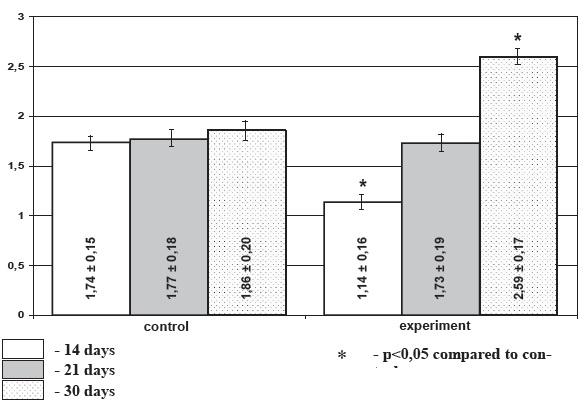
Diagram 2. Activation index of thyroid gland in chronic stress, М+/-m
Discussion and conclusion
Contradictory data regarding inhibitory or stimulator effect of stress on the thyroid gland and its follicular compartment were reported in some of the recent papers [4,7]. Quantitative evaluation of the follicular compartment of the thyroid gland in the present investigation demonstrated a distinct age-related pattern of the microstructural changes in the thyroid follicles under mild chronic stress conditions. These changes revealed inhibition of the functional activity of the thyroid gland in preweaning rats, its stimulation in the infant rats and a trend towards increased functional activity in the weaning rats. The data obtained provide evidence that responsiveness of the hypothalamo-hypophyseo-thyroid axis reveals distinct ontogenetic dynamics in the growing body of the experimental animals.
References
1. Khmel´nitskiĭ O.K., Khmel´nitskaia N.M., Tararak T.Ia., Vasil´eva N.A., Balykin M.V. Impact of intermittent hypobaric hypoxia on the thyroid morphofunctional state in experimental hyperthyroidism // Arkh Patol, 2006, 68(6):31-33.
2. Banu K.S., Aruldhas M.M. Sex steroids regulate TSH-induced thyroid growth during sexual maturation in Wistar rats // Exp Clin Endocrinol Diabetes, 2002,110: 37-42.
3. Banu K.S., Govindarajulu P., Aruldhas M.M. Testosterone and estradiol modulate TSH-binding in the thyrocytes of Wistar rats: influence of age and sex // J Steroid Biochem Mol Biol, 2001, 78, (4):329-342.
4. Kioukia-Fougia N., Antoniou K., Bekris S., Liapi C., Christofidis I., Papadopoulou-Daifoti Z. The effects of stress exposure on the hypothalamic-pituitary-adrenal axis, thymus, thyroid hormones and glucose levels // Prog. Neuropsychopharmacol. Biol. Psychiatry.- 2002.- Vol.26.- N5.- P.823-830.
5. Kmieć Z., Kotlarz G., Smiechowska B., Myśliwski A. The effect of fasting and refeeding on thyroid follicule structure and thyroid hormone levels in young and old rats // Arch Gerontol Geriatr, 1998, 26(2):161-175.
6. Mizokami T., Wu Li A., El-Kaissi S., Wall J.R. Stress and thyroid autoimmunity // Thyroid, 2004,14(12):1047-1055.
7. Pan Y., Kong L.D., Li Y.C., Xia X., Kung H.F., Jiang F.X. Icariin from Epimedium brevicornum attenuates chronic mild stress-induced behavioral and neuroendocrinological alterations in male Wistar rats // Pharmacol. Biochem. Behav. - 2007.- Vol.87.- N1.- P.130-140
8. Yin D., Tuthill D., Mufson R.A., Shi Y. Chronic restraint stress promotes lymphocyte apoptosis by modulating CD95 expression // J Exp Med, 2000, 191 (8):1423-1428.
Библиографическая ссылка
Degtyar Yu.V., Gupalo S.P., Sharaevskaya M.V., Kokin N.I., Khlebnikov Yu.V., Smirnova T.S., Kapitonova M.Yu. IMAGE ANALYSIS OF THE STRESS-RELATED CHANGES IN THE FOLLICLES OF THE THYROID GLAND // Международный журнал прикладных и фундаментальных исследований. 2009. № 2. С. 4-8;URL: https://applied-research.ru/ru/article/view?id=349 (дата обращения: 27.01.2026).

